Assessment of the use of dolomite for the recovery of ammonia from field landfill leachate
Project Details
- Student(s): Hussein Rayshouni
- Advisor(s): Mahmoud Wazne
- Department: Civil
- Academic Year(s): 2018-2019
Abstract
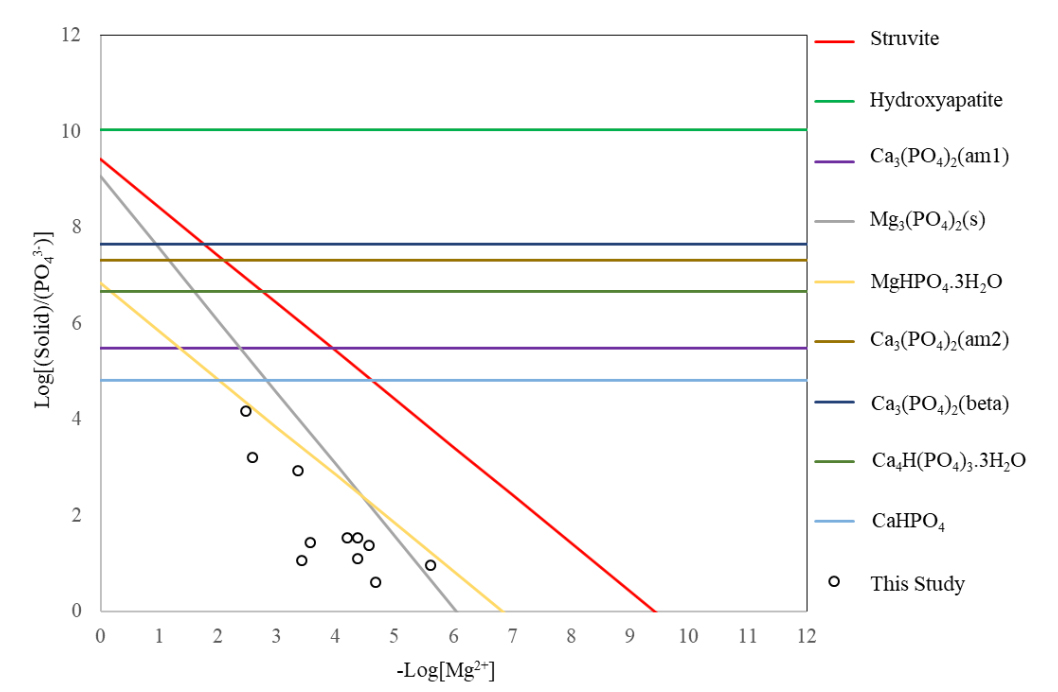
Ammonium in landfill leachates is a major contributor to environmental degradation if not effectively treated. However, it could be converted to a valuable fertilizer when it is co-precipitated with phosphate and magnesium as struvite. Low cost magnesium and phosphate sources are sought to offset the co-precipitation treatment costs, but most of the identified alternative magnesium sources have significant amounts of calcium, which may negatively impact the ammonium removal rates. In this study, the effects of calcium on ammonium removal from high strength aged field landfill leachate as struvite were investigated. Laboratory scale batch tests were conducted to assess the effects of pH, Mg2+:NH4+:PO43- and Ca2+:Mg2+ molar ratios on ammonium removal. Magnesium chloride salt was used as a model dissolved magnesium source whereas different compounds derived from dolomite [CaMg(CO3)2] were used as model solid phase magnesium sources. X-ray powder diffraction and activity ratio diagrams were used to delineate the ammonium removal mechanisms and struvite stability. The ammonium removal rate by the magnesium salt decreased from approximately 97% to 70% upon increasing the Ca2+:Mg2+ molar ratio from zero to 1.0, for Mg2+:NH4+:PO43- molar ratios of 1.25:1:1.25 and pH = 9.5. For similar pH values, and Mg2+:NH4+:PO43- and Ca2+:Mg2+ molar ratios, the ammonium removal rates by the dolomite derived compounds reached up to 55%, which highlighted the limited availability of magnesium in solid phases in addition to the negative impacts of calcium. The diffractometric analysis and thermodynamic calculations revealed the stability regions of struvite in the presence of competing solid phases. The new findings in this study could aid in the design of ammonium and phosphate removal and recovery systems by struvite precipitation.
Publications resulting from the thesis: